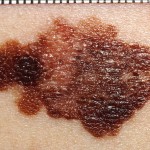
Melanoma treatment given a boost with newly approved medication
Melanoma treatment takes a step forward with the approval of the U.S. Food and Drug Administration of ipilimumab, manufactured by pharmaceutical firm Bristol-Myers Squibb Co.
The drug, marketed as Yervoy, is indicated for advanced melanoma patients. According to Bristol-Myers Squibb, the medication will cost $30,000 per dose or $120,000 for four-doses as part of a treatment regimen, which covers first-line melanoma treatment.
Yervoy belongs to a new class of anti-cancer drugs that helps the immune system destroy tumors. Melanoma is the most lethal kind of skin cancer and has been one of the most notorious cancers to treat. The current melanoma treatment includes chemotherapy using dacarbazine and interleukin-2, but neither has been effective in improving survival rates. Yervoy has been proven in clinical trials to extend the survival of melanoma patients, according to Bristol-Myers.
"Late-stage melanoma is devastating, with very few treatment options for patients, none of which previously prolonged a patient's life," said Richard Pazdur, who is director of the Office for Drug Evaluation and Research of the federal health agency's Center for Drug Evaluation and Research.
"Yervoy is the first therapy approved by the FDA to clearly demonstrate that patients with metastatic melanoma live longer by taking this treatment," added Pazdur in a statement.
It is expected that Yervoy will be administered with other drugs to further boost outcomes. Bristol-Myers also said it may test the drug for prostate cancer patients.
The clinical trial of 676 melanoma patients showed that those who used Yervoy had a median survival of 10 months compared to just 6.4 months for those who did not use the drug. The New England Journal of Medicine in August published the results of the study regarding the new melanoma treatment.