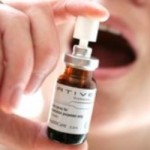
Cannabis Extract Spray Use Questioned By Medical Journal
A recently-published medical journal review found little proof to support using the cannabis extract spray for spasticity among multiple sclerosis patients.
However, GW Pharmaceuticals, the manufacturer of the cannabis extract spray, indicated that errors can be found on the report that gives a confusing observation on the drug. The report was published on the Drug and Therapeutic Bulletin, which is a publication of the British Medical Journal.
A GW partner, Bayer, sells the drug also called Sativex, which took GW over ten years to develop. British regulators have already given its approval for the use of the cannabis extract spray, which is an under-the-tongue spray, in 2010.
But DTB indicated that the proof backing the use of the drug was not enough to support its regular use. The review indicated that these drawbacks do not make it easy to support the clinical use of the drug.
The report goes against the opinion of twenty-two individual regulators in Europe and other parts of the world that have approved the use of the drug, according to GW Pharma.
The manufacturer also indicated that the Spanish and German medical reimbursement authorities have already given their approval for the drug. These two European countries require formal evaluations of the drug before they can be paid for.
Sales of Sativex in Britain have reached around $3.9 million or 2.4 million pounds for the past year until September 30. Total net sales have reached ten million pounds, which is higher than sales last year, which reached 5.3 million pounds.
The cannabis extract spray is available in a number of countries including Canada, Israel, Germany, Denmark, Britain, Spain, and Norway.